
The United States is locked in a quiet but heated race for bioscience and biotechnology supremacy with China. It is a race that changes everything. The winner will shape the health of the human population for generations to come, offer the power to greatly extend the number of healthy years people enjoy on this planet – and catalyze economic development at a scale roughly 100,000 times greater than mapping the human genome has. Few, if any, endeavors have the power to shape human destiny to such a profound extent.
The objective of this race is to find therapeutic drug candidates for the entire druggable proteome. Currently available drugs only address an estimated 670 of some 10,248 druggable proteins in the human body,[1] leaving a tremendous gap in humanity’s ability to treat diseases, defend against established and future novel pathogens, and slow age-related degeneration. Eliminating this gap and finding drug candidates for all possible targets will revolutionize the national – and global – state of medicine, whether addressing age-related diseases, naturally occurring infectious agents, or intentional acts of biological warfare. In fact, developing therapeutics for the entire human proteome will form an integral part of implementing the National Biodefense Strategy.[2]
What happens when it is possible to target the entire druggable human proteome?
- Precise targeting and control of non-infectious human diseases
- Agile development of host-targeted therapeutics protective against mutation-prone pathogens
- Extension of human life- and health-span
Addressing Non-infectious Disease
For starters, this capability will enable far more precise control over human disease than is possible today. Proteins give the human body structure and function. But some interactions between proteins facilitate various non-infectious diseases that inevitably occur during the course of the human lifespan. When these adverse interactions become runaway processes, disease progresses.
Although medicine today can intervene in certain disease processes with currently available drugs, addressing only a tiny fraction of the druggable proteome means that the existing set of treatments is woefully incomplete. Of some 10,000 diseases that affect humans, current FDA-approved medicines can only treat about 500 – often poorly, with a long list of side effects.
The treatments that are available typically do not offer precise control over disease processes. The drugs are often insufficiently specific: not only do they bind to and change the behavior of the targeted disease-associated protein, but they also adversely interfere with other proteins involved in healthy biological processes, causing a plethora of side effects. In addition, inhibiting the function of a disease-causing protein can result in undesirable up- or down-regulation of certain other proteins in the body. Without the availability of other drugs that could act in concert to maintain the desired balance, we lack the ability to intervene in a precise and personalized manner.
If novel drugs become available to precisely address various parts of the proteome, then medicine can control this game of protein target whack-a-mole. Precisely targeted therapeutic medicines can simultaneously adjust and retune the function of not only all the proteins implicated in a targeted disease process but also those proteins whose normal function should be preserved.
Agile Therapeutics Development & Production
The implications for public health are enormous – and go far beyond being able to treat every human disease better and more precisely. Being able to precisely target any functional site of all druggable proteins will also facilitate rapid development of therapeutics to address infectious diseases – whether naturally occurring, accidentally released, or intentionally developed as a weapon.
The typical approach to addressing infectious disease – particularly viruses that can cause pandemics like influenza or Covid – targets the pathogen itself. However, the propensity for viruses to mutate poses a significant challenge to this approach. Viruses can and do mutate to the point that they begin to evade immunity from vaccines and undergo other changes that render current antivirals less effective.
By contrast, the human proteome is a much more stable, static target. Viruses use proteins on the surfaces of human cells in order to gain entry and take over cellular machinery to produce more virions and spread. Drugs that target these cell-surface proteins and block viral entry are far less likely to lose efficacy as viruses evolve. And having drugs that target the human proteome in advance can serve as a first line of defense against entire classes of viruses as various novel viruses and new strains of older viruses emerge.
Healthspan and Lifespan Extension
Usually, people who fall victim to chronic age-related disease must wait until symptoms manifest to begin treatment. But being able to fine tune the human proteome will permit far earlier intervention while people’s bodies are simply in a state of disequilibrium leading toward illness instead of waiting until full-blown disease occurs. It will then be possible to delay the onset of disease and maintain the human body in a state of optimal function for much longer than is possible today. Combinations of certain drugs taken at intervals of months or years will even partially reprogram and rejuvenate cells, extending the state of youthful health and energy.
At that point, every person with access to such medical progress will enjoy more healthy, productive time on this planet than anyone does today. Turning 90 into the new 50 will give countries with access to these treatments the ability to dramatically expand their productive workforce – and create a manifold increase in the size of their economies.
What does it take to advance medicine to that point?
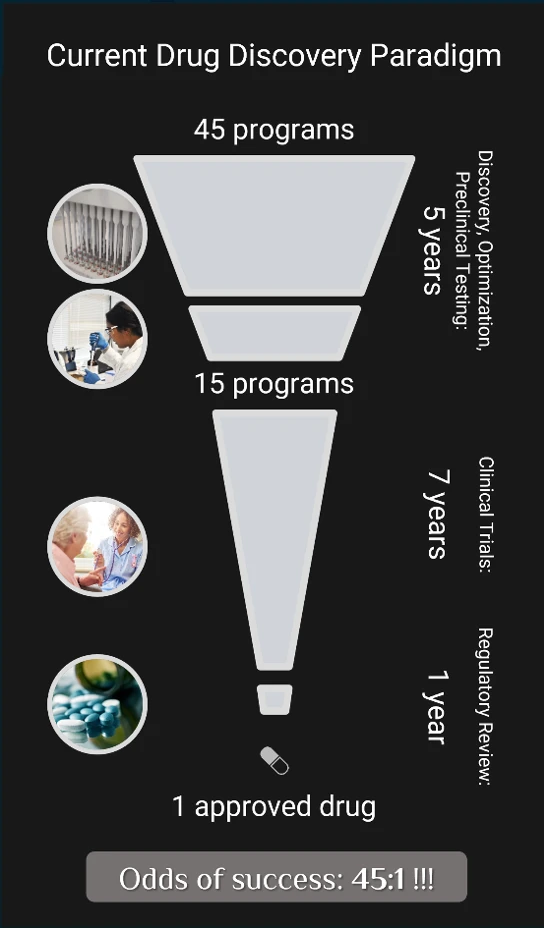
Achieving victory in the race for biotechnological supremacy requires overcoming a number of obstacles and is by no means a trivial task. After identifying the proteins implicated in specific disease processes, it is necessary to find the right small-molecule drug candidates that bind to those proteins. Using conventional trial-and-error testing to find drug candidates that bind to disease-associated proteins in a desired manner is a task with long odds of success. Under the current paradigm, two out of every three pharmaceutical programs fail to generate even one candidate viable for clinical trials. And overall, only one in forty-five drug discovery programs produces a drug that passes regulatory hurdles to reach market.
The main driver of failure in drug discovery is the daunting task of synthesizing and testing drug candidates in the lab. This task takes significant time to make relatively little progress: over the past 150 years, academia and the pharmaceutical industry have managed to make fewer than ten million out of a decillion (1033, or a one followed by 33 zeroes) chemotypes possible under the rules of organic chemistry. And AI alone is not the solution, since AI can only produce drug candidates similar to those on which it was trained – i.e., those for which experimental data already exists. Consequently, the truly novel breakthrough treatments of the future that lie in the unexplored parts of the chemical universe remain untapped.

Key components to overcoming these obstacles and successfully achieving biotechnological primacy include the following:
- Fundamental physics advances that allow atom by atom engineering of new molecules that lie in previously unexplored chemical space; molecules that precisely target disease-causing proteins while leaving desirable functions intact
- Highly automated chemistry and biology lab processes to characterize and validate these new molecules designed through molecular engineering
- AI that learns from all the new molecules being designed and tested to further accelerate drug discovery and development processes
- Large-scale testing on human organoids to increase the speed and safety of collecting data on how novel drugs will perform in vivo during clinical trials
- AI-driven, streamlined adaptive clinical trials augmented by supplementary test data from human organoids
- Domestically controlled, integrated, and automated continuous manufacturing processes that support the development and production of medicines while eliminating any supply chain bottlenecks
Fostering the right incentives and developing the right business model will unify these capabilities for maximum impact. Under the right model, research and development will systematically generate marketable drugs whose production flows seamlessly from computer models to the lab bench to full-scale automated manufacturing facilities. America’s leadership in bioscience and biotechnology hinges on its support of enterprises that can develop and integrate all the key components to success.
[1] https://www.icr.ac.uk/blogs/the-drug-discoverer/page-details/climbing-the-peaks-of-drug-discovery-tackling-the-most-challenging-targets-with-ingenuity-and-realism. Accessed September 24, 2024.
[2] https://www.whitehouse.gov/wp-content/uploads/2022/10/National-Biodefense-Strategy-and-Implementation-Plan-Final.pdf. Accessed September 24, 2024.

Adityo Prakash and Tom Cellucci
Adityo Prakash is the Chairman and CEO of Verseon International Corp. He started Verseon to change the way the world finds new medicines. His passion is building fundamental science-based solutions to major business problems that impact society. Prakash has led the development of Verseon’s drug discovery platform, novel drug pipeline, and overall business strategy. Under his leadership, Verseon has developed a variety of novel technologies to overcome the pitfalls of drug discovery and development. These advances include Deep Quantum Modeling™ (DQM) and VersAI™. DQM not only facilitates the design of drug structures inaccessible to the rest of the pharma industry but also models the interactions between these novel drug-like structures and target proteins with unprecedented accuracy. VersAI™ excels in making predictions based on the small, sparse datasets typical of the life sciences, demonstrating up to 35% greater accuracy than Google AutoML in peer-reviewed literature. These advances have helped Verseon to create a fast-growing pipeline of drug candidates whose uniquely desirable therapeutic profiles are poised to change the standard of care for heart attacks, strokes, diabetic end organ damage, cancers, and more. Previously, Prakash was the CEO and founder of Pulsent Corporation, where he was the primary inventor of technology at the heart of all video streaming today. With a track record of delivering industry firsts, he is an inventor on 40 patent families. Prakash received his BS in physics and mathematics from Caltech. || H.E. The Hon, Sir Dr. Thomas A. Cellucci, PhD, MBA is a serial entrepreneur, currently managing several high-tech firms. He was appointed the US Department of Homeland Security’s Director of the Research & Development Partnerships (RDP) Group managing over $12B in assets and over 1700 team members. He was also the first Chief Commercialization Officer in the US Federal Executive Branch and continues to assist the President of the United States and Chairman of the Joint Chiefs. In 1999, he founded Cellucci Associates, Inc. with headquarters at Harvard Square in Cambridge, MA. Cellucci writes about the intersection of emerging technology, commercialization, and implementation to protect the homeland. Cellucci earned a PhD in Physical Chemistry from the University of Pennsylvania (1984), an MBA from Rutgers University (1991), and a BS in Chemistry with Honors from Fordham University (1980). He holds two endowed Chairs at prestigious universities in Kazakhstan and has taught at Harvard Business School, Princeton University, and the University of Pennsylvania.
